Molar Mass of Sulphur
A change in internal energy can be expressed as. Molar Atomic mass g mol 1.

Icse Solutions For Class 10 Chemistry Mole Concept And Stoichiometry
Chemical Reaction Calculator.

. C v specific heat for gas in a constant volume process kJkgK. By calculation the molar mass of acetic acid comes out to be 6005 gmol. Molar Volume Calculator.
A Cimetidine B Ranitidine C Histamine D Saccharin. By NTA D Allen Ans. If both nitrogen and sulphur are present in an organic compound then the excess of sodium used in sodium fusion will decompose the sodium thiocyanate formed to give NaCN and Na 2 S.
Chemical Equation Calculator. We further estimate that a global mass of 1945 1015 grams of organic carbon is preserved in surface marine sediments as a result of its association with iron4. Ideal gas equation Molar mass Molar volume method Mole Mole concept Molecular weight Molecule Number of moles One gram atom Regnaults method Vapour density Victor Meyers method.
Acetic Acid Melting Point. Sulphur is generally shipped in bulk as well as in Jumbo bags but sulphur powder usually is packed in 25-50kg bags due to its nature and being a flammable material. For comparison the molar mass of air which is about 80 nitrogen and 20 oxygen is approximately 30 gmol which leads to a speed.
Molar Mass Definition Unit Mole Mass vs Atomic Mass Atomic Weight Molar of Mixtures Examples and Calculations. Sulfur hexafluoride SF 6 or sulphur hexafluoride British spelling. 1 A 35 B 50 C 75 D 125.
Of sulphur Wtof. Metals and semimetals common liquids and fluids and other common substances as well as values of molar heat capacity of common organic substances and inorganic. Molality moles of solute kilograms of solvent.
Molality m is defined as the number of moles of solute per kilogram of solvent. Molecular mass of methane SO 2 32 1 16 2 32 32 64 g. And therefore molarity will be 1.
In short the chemical equation should have a balanced mass and charge on both sides of the reaction. An online atomic mass calculator allows you to find the atomic mass and number atomic symbol and stability of any element with number of neutrons and protons. Du c v dT 1 where.
Q1Calculate the molar mass of the following substancesa Ethyne C2H2b Sulphur molecule S8c Phosphorus molecule P4Atomic mass of phosphorus 31d Hydrochloric acid HCle Nitric acid HNO3AnswerMolar mass of C 12gMolar mass of H 1gMolar mass of S 32gMolar mass of P 31gMolar mass. Molar Atomic mass g mol 1. H NH 2 Histamine N N.
0 16. R 0082 L atm K 1 mol. Coupling Reaction - Types Example and Applications.
The value is given in gmole or gramsmole. It is abundant multivalent and nonmetallicUnder normal conditions sulfur atoms form cyclic octatomic molecules with a chemical formula S 8Elemental sulfur is a bright yellow crystalline solid at room temperature. By NTA C Allen Ans.
The molar mass of the gas in g mol 1. 9 6 g m l molecular weight of H 2 S O 4 9 8 Take reaction S O 3 H 2 O H 2 S O 4. You can determine.
C 12 A 1400 B 2200. 354444 g mol 1. Fe 56 Molar Atomic mass g mol 1.
Light yellow flakes crystals or powder. For an ideal gas the internal energy - u - is a function of temperature. Electric Heating of Mass - Electric heating of an object or.
Specific heat c v varies with temperature but within moderate temperature changes the. Molecular mass of Sulphuric acid is 98. Now if 98 gram of sulphuric acid is present in 1 litre of solution then it is 1 molar solution of Sulphuric acid.
Difference Between Mass and Matter. Sulphur iodine and many other organic compounds are dissolved in it. Molar Mass of Reineckes Salt C 4 H 12 N 7 OCrS 4.
Du change in internal energy kJkg. Sulfur or sulphur in British English is a chemical element with the symbol S and atomic number 16. By knowing the initial mass of the fuel sample the heating value of the sample can be calculated by dividing the heat released by the mass of the sample.
Same for both the sides reactant and product of the equation. DT change in temperature K. Sulfur is the tenth most abundant element by mass in the universe.
Disulphur Bromine Sulphur Dibromide The reaction type. 64 10-2 kg of sulphur dioxide. Contain sulphur atom.
Standard molar entropy S m o Song et al 2011 and heat capacity C p Rath et al 2003 of dry biomass were predicted via Eq. 7 and Eq8 respectively. The melting point of acetic acid is 2895K or 165C.
6 has a molar mass of about 146 gmol and the speed of sound through the gas is about 134 ms at room temperature pitching the voice down. Calculate the mass percent ww of sulphuric acid in a solution prepared by dissolving 4 g of sulphur trioxide in a 1 0 0 ml sulphuric acid solution containing 8 0 mass percent ww of H 2 S O 4 and having a density of 1. The molar mass of a compound or an element is the mass per mole of the substance.

What Is Enthalpy Of Reaction A Plus Topper Https Www Aplustopper Com Enthalpy Reaction Heat Reaction What Is Th Molar Mass Reactions Chemical Equation

A Chemist Reviews A Book Chemistry Humor Science Cartoons Science Humor
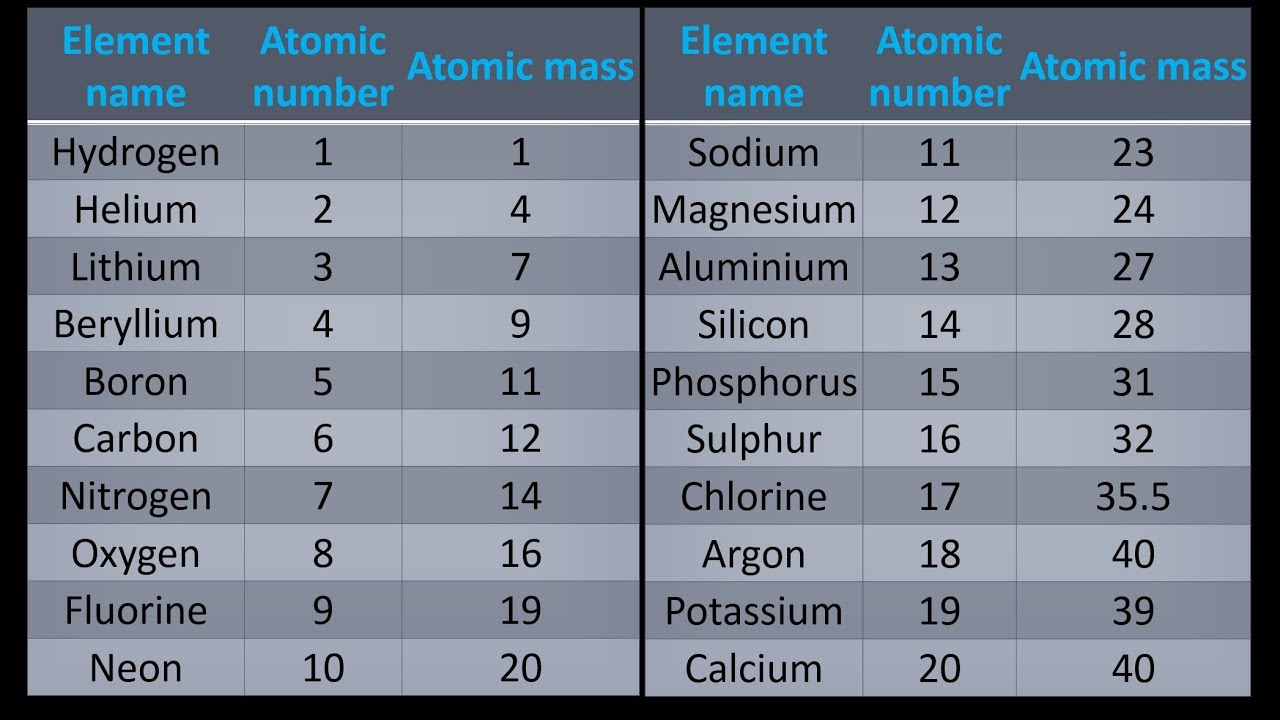
A Simple Way To Get Atomic Mass Of First 20 Elements Of The Periodic Table Youtube Chemistry Lessons Element Chemistry Chemistry Basics

How Does The Metal Reactivity Series Work Example Chemistry Lessons Study Chemistry Biology Facts

Sulphur Dioxide So2 Sulphur Dioxide Molecular Geometry Sulphur

0 Response to "Molar Mass of Sulphur"
Post a Comment